
Kód: 09193151
Benefit-Risk Assessment of Medicines
Autor James Leong, Sam Salek, Stuart Walker
Over the past decade pharmaceutical companies and regulatory agencies have been reviewing the benefit-risk assessment of medicines with a view to developing a structured systematic standardized approach. In view of this a universa ... celý popis
- Jazyk:
 Angličtina
Angličtina - Väzba: Pevná
- Počet strán: 317
Nakladateľ: Springer International Publishing AG, 2015
- Viac informácií o knihe

62.70 €

Skladom u dodávateľa v malom množstve
Odosielame za 10 - 15 dní
Potrebujete viac kusov?Ak máte záujem o viac kusov, preverte, prosím, najprv dostupnosť titulu na našej zákazníckej podpore.
Pridať medzi želanie
Mohlo by sa vám tiež páčiť
-

Nearing Home! Comforts and Counsels for the Aged
15.02 € -2 % -

Day This Lit
17.49 € -

Transformative Urban Open Space
18.42 € -

L'Ame De Fond
15.13 € -

Vorlesungen über allgemeine Pathologie
38.40 € -9 % -

Bedeutung von Events fur den Tourismus
59.09 € -2 % -

Medium Film im Geschichtsunterricht
26.87 € -1 %
Darujte túto knihu ešte dnes
- Objednajte knihu a vyberte Zaslať ako darček.
- Obratom obdržíte darovací poukaz na knihu, ktorý môžete ihneď odovzdať obdarovanému.
- Knihu zašleme na adresu obdarovaného, o nič sa nestaráte.
Viac informácií o knihe Benefit-Risk Assessment of Medicines
Nákupom získate 154 bodov
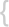 Anotácia knihy
Anotácia knihy
Over the past decade pharmaceutical companies and regulatory agencies have been reviewing the benefit-risk assessment of medicines with a view to developing a structured systematic standardized approach. In view of this a universal framework together with an appropriate documentation system for recording benefit-risk decisions has been developed and applied and is a valuable addition to the Benefit-Risk Toolbox. The opportunity to examine the implementation of this approach by several regulatory authorities places this book in a unique position with the potential to positively contribute to the ongoing debate in this area.§§The field of benefit-risk assessment continues to evolve at a rapid pace due to political and societal pressure, as is also reflected in the recent FDA PUDFA agreement as well as in the EMA 2015 Roadmap. Therefore, this is not an attempt to provide a comprehensive and complete account of the current benefit-risk assessment environment; rather, the strength of this book is that it provides an evaluation of some current approaches to the benefit-risk assessment of medicines and outlines the development of a new universal framework. It also demonstrates how a number of mature agencies have implemented this universal framework together with the methodology for documenting their benefit-risk decisions. In addition, it also reviews current publicly available documents together with their strengths and weaknesses in communicating benefit-risk decisions to stakeholders.§§
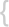 Parametre knihy
Parametre knihy
Zaradenie knihy Knihy po anglicky Medicine Medicine: general issues Public health & preventive medicine
62.70 €
- Celý názov: Benefit-Risk Assessment of Medicines
- Podnázov: The Development and Application of a Universal Framework for Decision-Making and Effective Communication
- Autor: James Leong, Sam Salek, Stuart Walker
- Jazyk:
 Angličtina
Angličtina - Väzba: Pevná
- Počet strán: 317
- EAN: 9783319158044
- ISBN: 331915804X
- ID: 09193151
- Nakladateľ: Springer International Publishing AG
- Hmotnosť: 638 g
- Rozmery: 244 × 168 × 25 mm
- Dátum vydania: 05. May 2015
Obľúbené z iného súdka
-

Why We Eat (Too Much)
11.42 € -21 % -

Glucose Revolution
17.60 € -29 % -

The Art & Science of Foodpairing
42 € -15 % -

China Study: Revised and Expanded Edition
16.36 € -13 % -

Encyclopedia of Bach Flower Therapy
28.62 € -21 % -

Miller's Review of Critical Vaccine Studies
18.83 € -19 % -

Nutrition and Physical Degeneration
37.16 € -

Rewire Your Anxious Brain
17.70 € -13 % -

Energy Paradox
23.88 € -17 % -

Decision Modelling for Health Economic Evaluation
82.98 € -

Gordis Epidemiology
54.77 € -1 % -

Cure Tooth Decay
37.47 € -

Motivational Interviewing in Nutrition and Fitness
38.09 € -

Salt Fix
15.54 € -28 % -

Yes, You Can Get Pregnant
16.57 € -18 % -

After Cancer Care
15.02 € -23 % -

Gut
9.05 € -28 % -

Nutrition and Physical Degeneration
39.22 € -

Oxford Handbook of Clinical Medicine
62.08 € -

It's All in Your Head
11.01 € -24 % -

Close Your Mouth
13.27 € -23 % -
Neurosonology and Neuroimaging of Stroke
173.91 € -14 % -

Manual of Dietetic Practice 6e & Dietetic Case Studies Set
149.71 € -5 % -

Worry Trick
16.98 € -20 % -

Body Awareness as Healing Therapy
13.89 € -20 % -

Bio-identical Hormones and Telomerase
25.22 € -4 % -

Inner Level
11.52 € -28 % -

I Think You'll Find It's a Bit More Complicated Than That
14.41 € -23 % -

Cancer Revolution
15.02 € -30 % -

Evidence-Based Medicine
42.10 € -6 % -

Causal Inference for Statistics, Social, and Biomedical Sciences
65.99 € -2 % -

Never Too Late to go Vegan
15.33 € -17 % -

Integrative and Functional Medical Nutrition Therapy
151.77 € -4 % -

Nutritional Psychiatry
50.03 € -

Nutrition for Sport and Exercise - A Practical Guide
62.60 € -

Nutrition for Sport, Fitness and Health
134.78 € -9 % -

Nutrition Essentials for Mental Health
50.24 € -2 % -

Mikronährstoff-Räuber: Metformin
5.14 € -

Clinical Studies in Medical Biochemistry
70.01 € -

Marijuana Medicine
26.45 € -4 % -

Galen on Food and Diet
65.89 € -

Marketing Nutrition
140.65 € -

Oxford Handbook of Applied Bayesian Analysis
82.98 € -
WHO classification of tumours of haematopoietic and lymphoid tissues
227.66 € -

Bella Figura
9.46 € -28 % -

Biofeedback
94.10 € -

Proteinaholic
13.68 € -14 % -

Vaccines, Autoimmunity, and the Changing Nature of Childhood Illness
19.66 € -28 % -

Divided Mind
18.32 € -18 %
Osobný odber Bratislava a 2642 dalších
Copyright ©2008-24 najlacnejsie-knihy.sk Všetky práva vyhradenéSúkromieCookies


 21 miliónov titulov
21 miliónov titulov Vrátenie do mesiaca
Vrátenie do mesiaca 02/210 210 99 (8-15.30h)
02/210 210 99 (8-15.30h)